Activities
Chlorofluorocarbons
Chlorofluorocarbons (CFCs) are carbon compounds containing fluorine and chlorine atoms. They were produced by the man for a wide variety of applications. The most common are CFC-11 and CFC-12. CFC-11 (CCl3F) was principally used as blowing agent for foams and packaging materials, and as refrigerant in large commercial chillers. CFC-12 (CCl2F2), known as Freon-12, was also used in air conditioning. CFCs are photodissociated by the ultraviolet radiation in the stratosphere, where chlorine is released. The increase of stratospheric chlorine concentration associated with CFC emissions is responsable for the stratospheric ozone depletion phenomenon. CFCs are important greenhouse gases; their net 100-year global warming potential is 4600 and 10600 for CFC-11 and CFC-12 respectively [IPCC 2001].
The fast increase of the CFCs emissions in the last years, together with the discovery of the Antarctic ozone hole led to the formulation of the Montreal Protocol
[1987] and its Amendments, which regulate the production of ozone-depleting compounds and called for the elimination of CFC production by 1996.
Measurements of CFCs concentration have been started at Lampedusa in 1997. Monthly mean concentrations
of CFC-11 and CFC-12 at Lampedusa, and at other stations in the Northern hemisphere, are displayed in figures 1 and 2. The concentration of CFC-11 reached a maximum
in 1993; CFC-12 concentration recently stabilized around 540 pptv. This behavior depends on the long halocarbon atmospheric lifetime (50 and 102 years respectively
for CFC-11 and CFC-12), and on the time needed to curtail emissions.
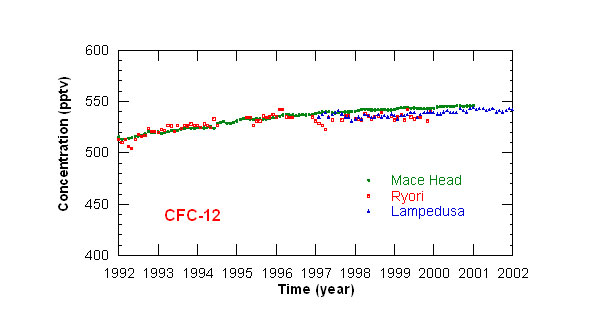
Figure 2: time series of monthly mean CFC-12 concentrations
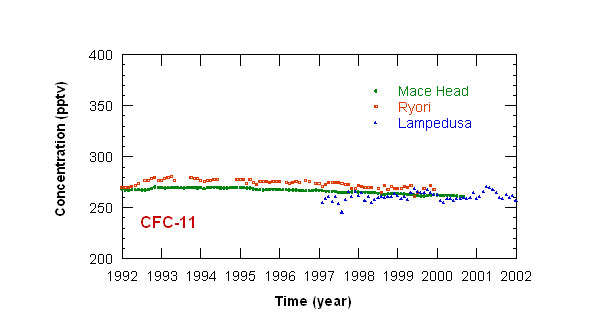
Figure 1: time series of monthly mean CFC-11 concentrations
